
Medicines & Healthcare Products Regulatory Agency - PharSafer® - Specialists in Global Clinical and Post Marketing Drug Safety
Guidance for UK Manufacturer's Licence and Manufacturer's Authorisation (for Investigational Medicinal Products) Holders on
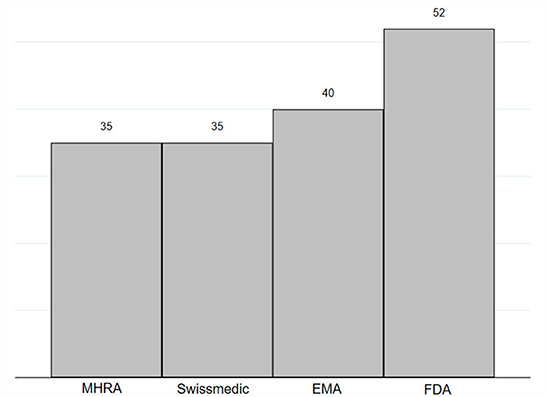
Frontiers | Regulatory policy and pharmaceutical innovation in the United Kingdom after Brexit: Initial insights











![Free MHRA citation generator [2022 Update] - BibGuru Free MHRA citation generator [2022 Update] - BibGuru](https://www.bibguru.com/img/mhra-book.png)




