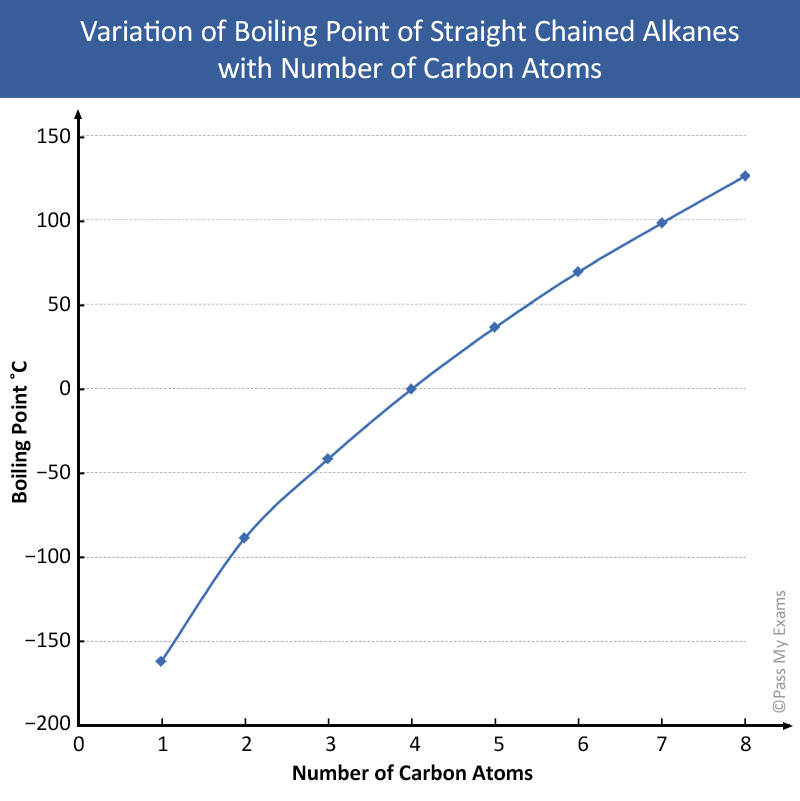
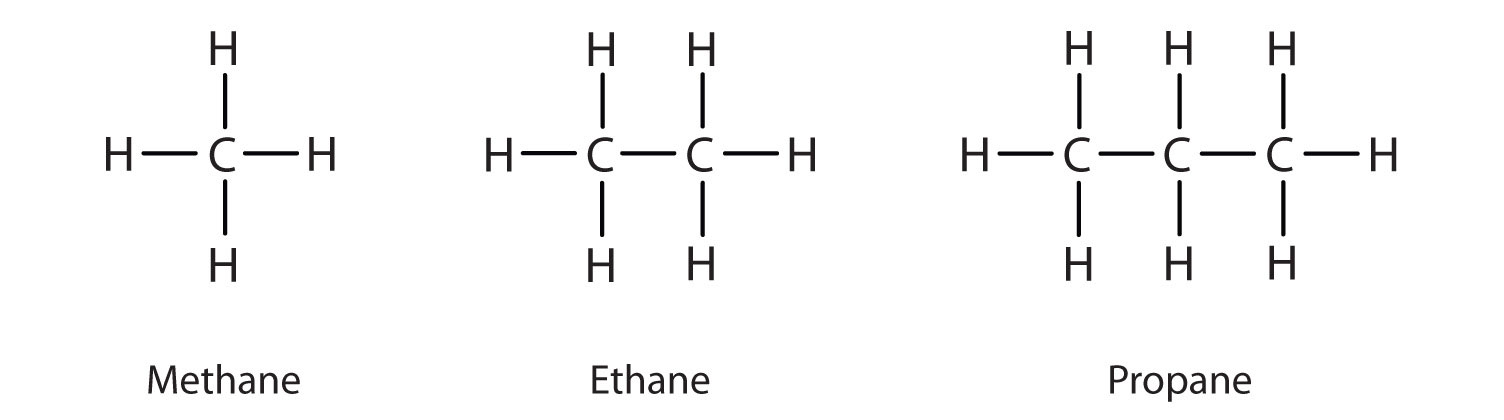
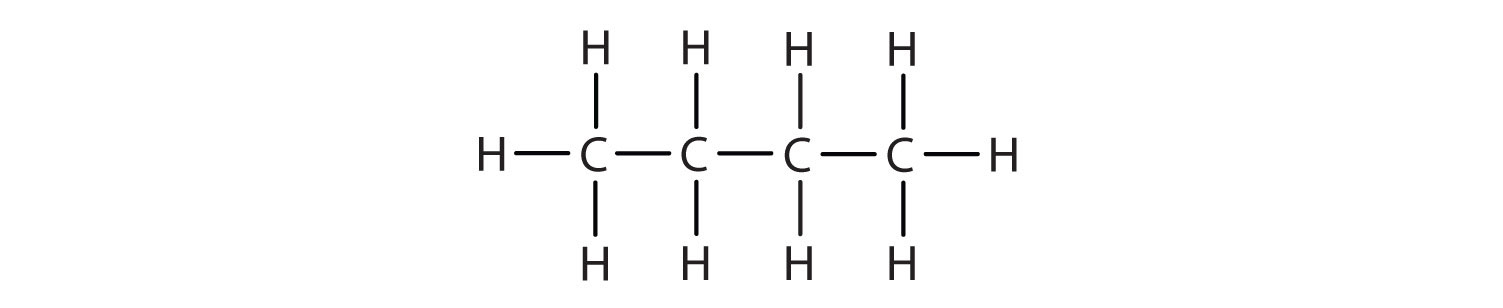
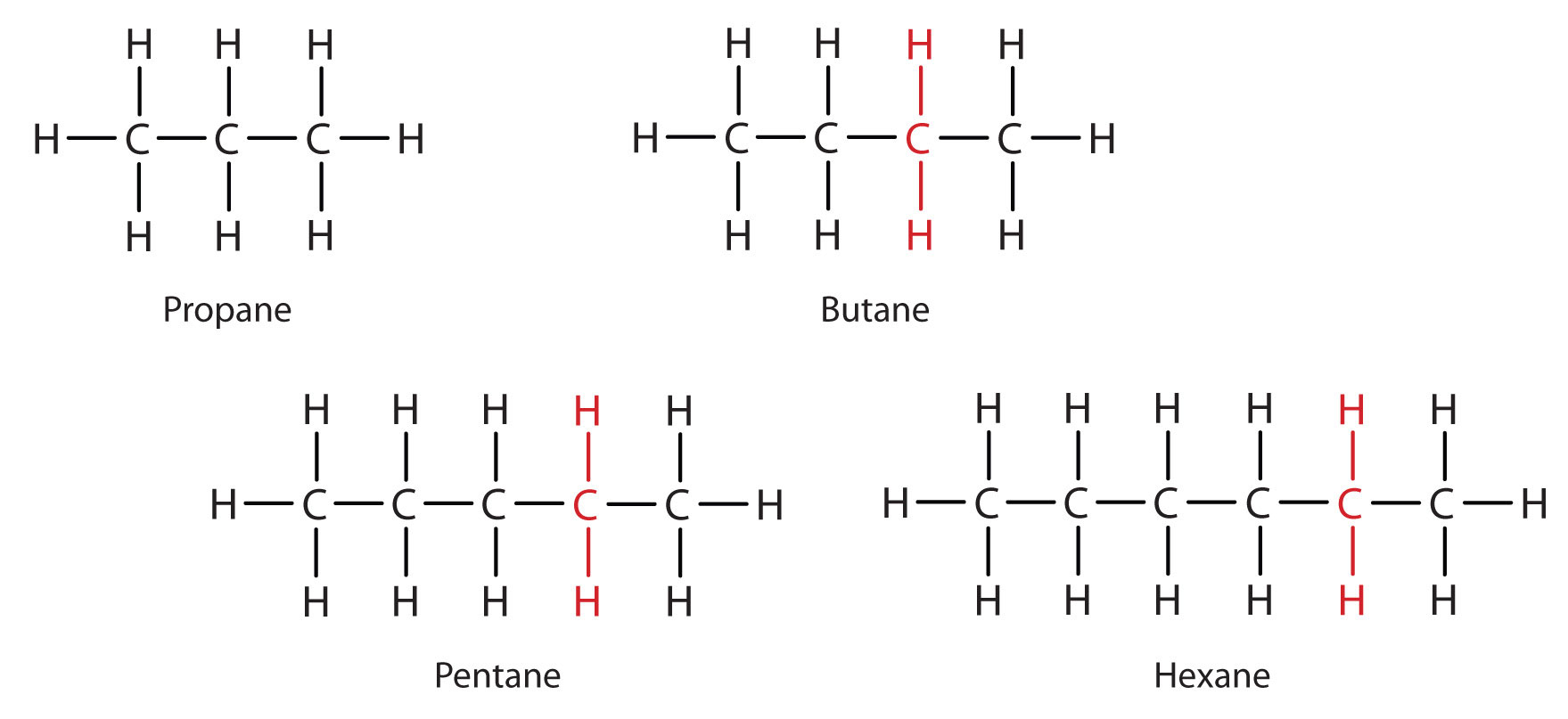
a Give the general formula of alkanes. Write the name, structural formula and physical state of the compound containing:i 3 carbon atomsii 8 carbon atomsb Which of the compounds named in a

A hydrocarbon molecule has 3 carbon atoms. Write down its molecular formula if it is an : (i) alkane , (ii) alkene, (iii) alkyne.

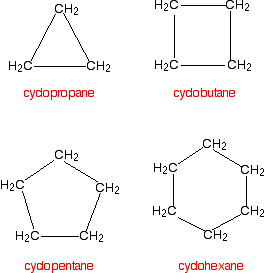
Organic Chemistry Nomenclature: Cyclic Alkanes. Cycloalkanes Cycloalkanes: An alkane that contains a ring of carbon atoms. Ring sizes from 3 carbons to. - ppt download
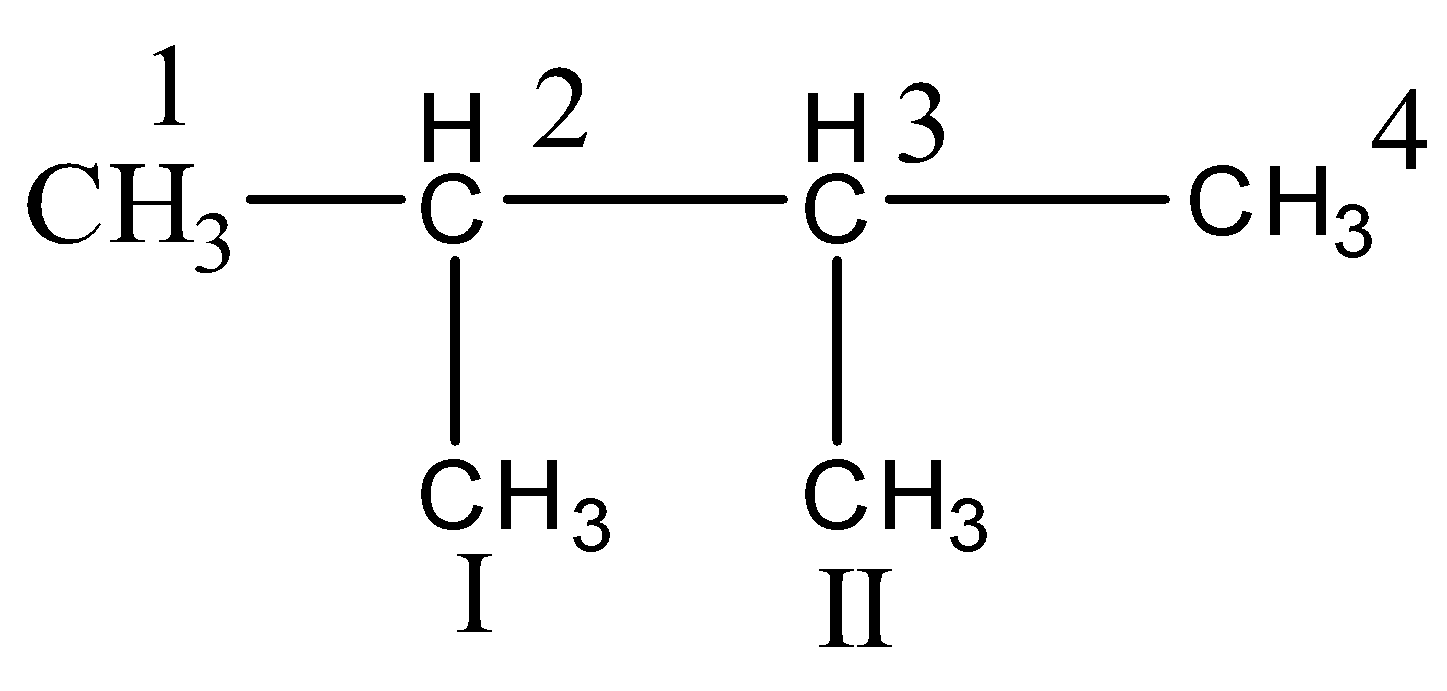
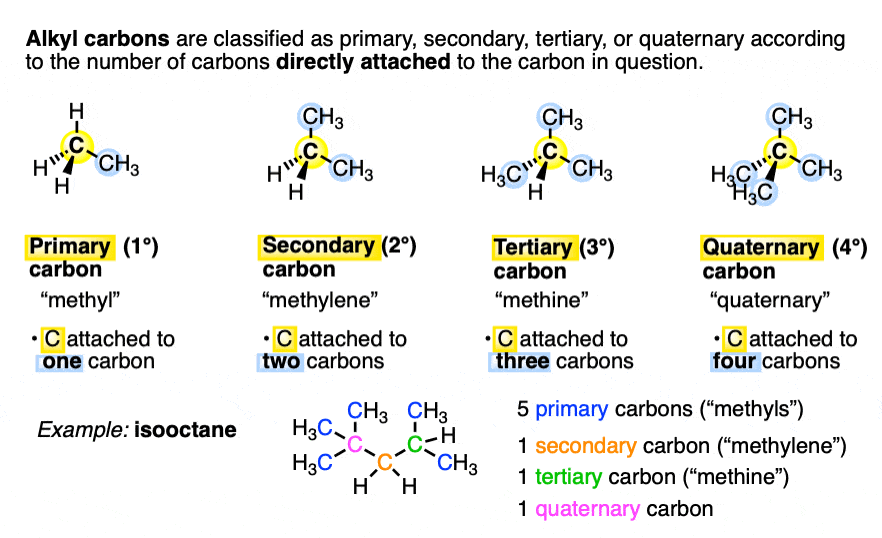
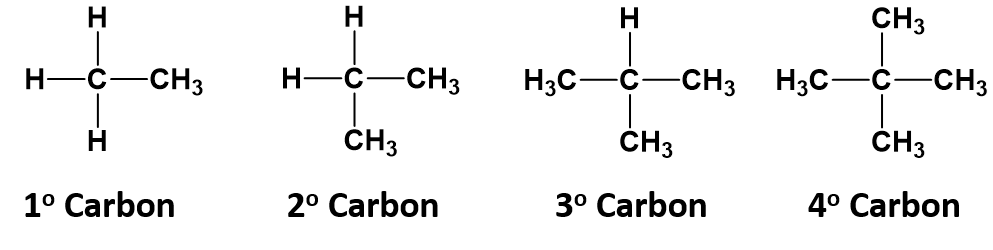
which alkane would have only primary and tertiary carbon?(A) Pentene(B) $ 2 - $ methyl butane(C) $ 2,2 - $ dimethylpropane(D) $ 2,3 - $ dimethyl butane

How would you draw an alkane that has more than 3 but less than 10 carbon atoms and only primary hydrogens? | Socratic

IGCSE Chemistry: 3.3 draw displayed formulae for alkanes with up to five carbon atoms in a molecule, and name the straight-chain isomers